Acetaminophen toxicity
Background
- Recommended maximum total daily dose:
- Adults: 4gm/day
- Peds: 75mg/kg/day
- Toxic dose
- >10gm or >200mg/kg as single ingestion or over 24hr period OR
- >6gm or >150mg/kg per 24hr period x2d
- 200 mg/kg in healthy children 1-6 yoa
- Peak serum levels seen within 2hr
The 150 Rule
- Toxic dose is 150 mg/kg
- Give NAC if level is >150 mcg/mL four hours post-ingestion
- Initial loading dose of NAC is 150 mg/kg IV (140mg/kg PO)
Pharmacology
Mechanism of action
- Poorly understood
- Possibly through inhibition of Cyclooxygenase-3 (COX-3)
- Decreases synthesis of prostaglandins
- Antipyresis through inhibition of hypothalamic heat center
Pharmacokinetics
- A - Rapid and near complete absorption
- D - Vd = 0.95 L/kg
- M - T 1/2 = 1.5-2hrs
- 40-60% - Glucuronidation
- 20-40% - Sulfuronidation
- 5-10% - Metabolism through CYP450 (Forms NAPQI)[1]
- E - Conjugated and unconjugated excreted through kidneys
Toxicology
Pathophysiology
- APAP toxic metabolite NAPQI usually quickly detoxified by glutathione stores in liver
- In overdose, glutathione runs out, NAPQI accumulates -> liver injury
- NAC increases availability of glutathione
- NAC is a precursor
Clinical Features
- Stage 1 (first 24hr)
- Mild N/V/malaise
- Hypokalemia (a/w high 4-hr level)
- Stage 2 (days 2-3)
- Improvement in symptoms
- RUQ abd pain
- Elevated transaminases
- Elevated bilirubin, PT (if severe)
- Stage 3 (days 3-4)
- Recurrence of N/V
- Hepatic failure
- Jaundice
- Coagulopathy
- Encephalopathy (esp w/ massive ingestions)
- Renal failure (1-2%; usually after hepatic failure is evident)
- Pancreatitis (rare)
- Stage 4 (after day 5)
- Clinical improvement and recovery (7-8d) OR
- Deterioration to multi-organ failure and death OR
- Continued deterioration
Work-Up
- APAP level
- Chemistry
- Metabolic acidos seen w/ extremely large ingestion
- LFT
- PT/PTT/INR
- Acetaminophen level: 4 hours post ingestion and repeat in 4 hours
- ASA levels and other co-ingestants
Diagnosis
- APAP level
- Obtain 4hrs post-ingestion
- Obtaining multiple levels is rarely indicated in the absence of hepatotoxicity
- Nomogram (see below)
- Only indicated for single, acute ingestion occurring <24hr prior to presentation
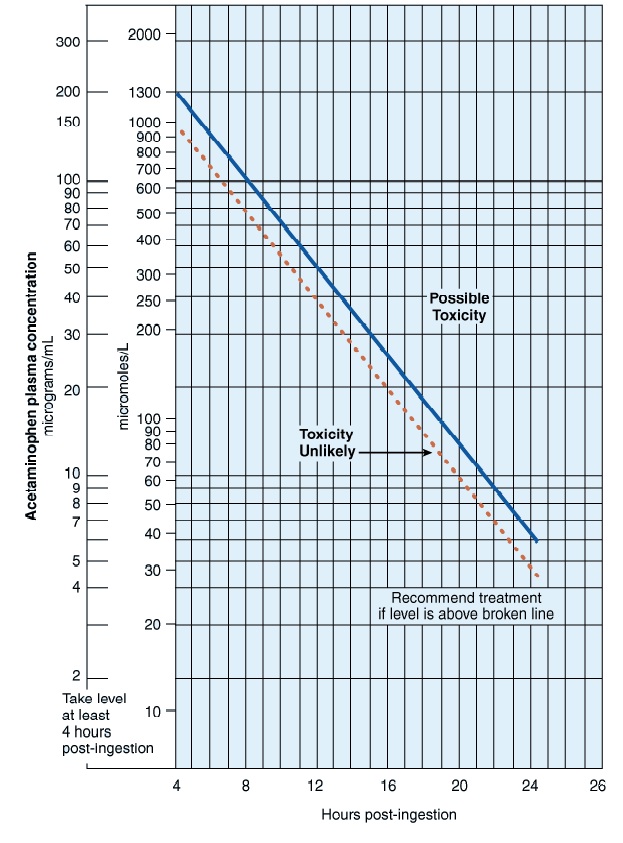
Rumack-Matthew Nomogram
- Not useful for chronic ingestion (patients who take supratherapeutic doses for several days) or if time of ingestion is unknown
- Make sure you use the correct units!
Treatment
- Very important to identify time of ingestion
<4hr after ingestion
- GI decontamination
- Activated Charcoal if <3 hr post-ingestion (no role for multidose activated charcoal)
- Gastric Lavage if high-morbidity coingestants and <1 hr post-ingestion
- Send 4hr APAP level
- Toxic level: Give NAC
- Nontoxic level: No treatment necessary
Between 4-24hr after ingestion
- Send APAP level
- If level will be available within 8hr post-ingestion: wait for level before treating
- If level will not be available within 8hr post-ingestion: do not wait for level before treating
- Discontinue treatment if level returns non-toxic
Unknown or >24hr after ingestion
- Consider GI decontamination for unknown ingestion time
- Give 1st dose of NAC
- Send APAP level, LFT, coags
- APAP level >10 OR elevated transaminases? If yes then continue NAC
- pH <7.3 or PT >100 or Cr >3.3 or AMS? If yes refer to liver transplant unit
- APAP level and LFT both normal? If yes then stop NAC (treatment not indicated)
- APAP level >10 OR elevated transaminases? If yes then continue NAC
Chronic Ingestion
- Initiate NAC in any patient with evidence of ongoing hepatotoxicity (lft abnormalities) OR 'positive' tylenol level (>20 mcg/mL)
- If patient has normal lfts and 'negative' tylenol levels (>20 mcg/mL) do not require NAC treatment
Overdose in Pregnancy
- Both IV or oral NAC may be used in pregnant patients with Acetaminophen toxicity. [2]
- IV formulation may be preferred to increase fetal NAC concentrations
Extended release overdose
- Extended-release acetaminophen (Tylenol ER) consists of acetaminophen 325 mg in immediate release (IR) form surrounding a matrix of acetaminophen 325 mg
- Several studies show that the elimination of ER and IR APAP preparations is nearly identical after 4 hours. However, some case reports have documented APAP levels that are above the potential toxicity and treatment line on the nomogram as late as 11-14 hours after the ingestion of the ER preparation.
- Recommended management includes the measurement of 4-, 6-, and 8-hour APAP concentrations. Begin NAC therapy if any level crosses above the nomogram treatment line. If the 6-hour level is greater than the 4-hour level, begin NAC therapy.
N-acetylcysteine (NAC)
Background
- Almost 100% effective if given <8 hr post-ingestion; less effective if 16-24 hr post-ingestion
- May still be useful >24 hr post-ingestion, even with fulminant hepatic failure. Give NAC until LFTs improve (not until APAP level is 0) [3] [4]
- Be aware NAC treatment may affect PT. May see a dose-dependent increase in PT following NAC in patients without hepatotoxicity. [5]
Dosing:
PO
- 140mg/kg PO load
- 70mg/kg PO q4hr x17 doses additional; dilute to 5% soln
- Side Effects
- sulfur-smell causes nausea and vomiting. Consider mixing with juice or soda, in a cup with a lid and straw
IV
- Loading dose: 150mg/kg in 100 mL D5W over 60min
- Second (maintenance) dose: 50mg/kg in 250 mL D5W over 4hr
- Third dose: 100mg/kg in 500 mL D5W over 16hr
- Side Effects
- Anaphylactoid reaction but also associated with seizures, cerebral edema, & herniation. [6]
- Anaphylaxis responds to standard therapies and can usually restart NAC without safely without complications. [7]
King's College Criteria
- criteria for predicting fulminant hepatic failure, and thus referral to transplant center
- PPV 70-90% and sensitivity 69%
- includes:
- pH<7.3 or lactate>3 at 12hrs after full fluid resuscitation, OR all of the following:
- Cr>3.4
- INR>6.5
- grade 3 or 4 Hepatic Encephalopathy
- other predictors of APAP-induced hepatic failure include:
- lactate>3.5 4hrs after fluid resusciation
- phos>3.8 at 48hrs, OR
- APACHE II >15
Disposition
- Consider discharge for asymptomatic pts who do not require NAC
- Admission if requiring NAC or other ingestions, injuries
- Transfer to transplant center based on above criteria
- Psych consult if pt has suicidal ideation
External Links
- MDCalc - Acetaminophen Overdose & NAC Dosing
- MDCalc - King's College Criteria for Acetaminophen Toxicity
References
- ↑ Hendrickson RG, Bizovi KE. Acetaminophen. In: Flomenbaum NE, Goldfrank LR, Hoffman RS, et al, eds. Goldfrank’s Toxicologic Emergencies. 8th ed. New York: McGraw-Hill; 2002:523-543. (Textbook chapter)
- ↑ Heard KJ. Acetylcysteine for acetaminophen poisoning. N Eng J Med. 2008;359(3):285-292. (Review)
- ↑ Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol-induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303(6809):1026-1029. (Prospective randomized controlled trial; 50 patients)
- ↑ Harrison PM, Keays R, Bray GP, et al. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of N-acetylcysteine. Lancet. 1990;335(8705):1572- 1573. (Retrospective analysis; 100 patients)
- ↑ Wasserman GS, Garg U. Intravenous administration of Nacetylcysteine: interference with coagulopathy testing. Ann Emerg Med. 2004;44(5):546-547. (Letter)
- ↑ http://journals.lww.com/em-news/Fulltext/2012/02000/Toxicology_Rounds__Lessons_from_the_Courtroom_.9.aspx
- ↑ Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol (Phila). 2009;47(2):81-88. (Systematic literature review)